Classifying Diamond
Diamond can be classified into two major origins: (1) natural diamond, and (2) synthetic diamond.
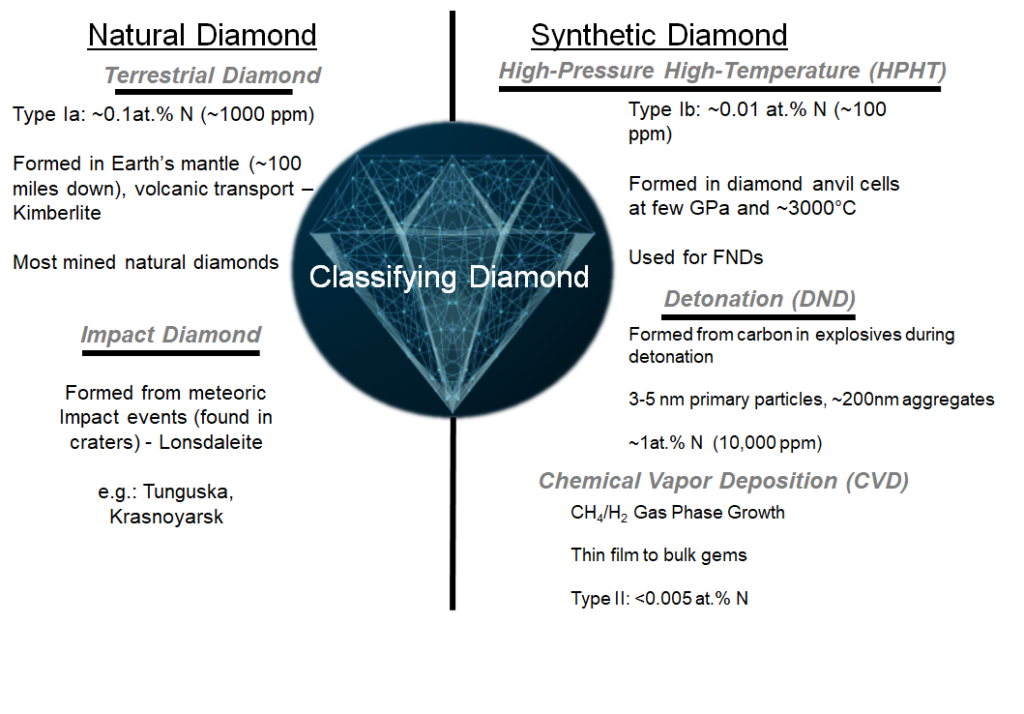
Natural Diamond
Natural diamond consists of any diamond formed as a result of naturally occurring processes or events. Within the natural diamond classification, two major sub-classifications can be identified, which we will refer to as terrestrial and impact diamond.
Terrestrial diamond is formed within the Earth’s mantle (~100 miles below the surface) where incredible temperatures and pressures can facilitate the formation of diamond from carbon material. These diamonds are transported to the surface via volcanic transport, and are typically found within the mineral Kimberlite. Mined natural diamonds are primarily sourced from Kimberlite.
Impact diamond is formed as a result of meteoric impact events, and can be found within the craters of impact sites. The high temperatures and pressures of the impact can facilitate the formation of diamond. One interesting mineral resulting from impact events is Lonsdaleite (or hexagonal diamond), which is a particularly unique allotrope of carbon with a different crystallographic form as compared to traditional cubic diamond.
Synthetic Diamond
Synthetic diamond consists of diamond grown via human developed methods. The development of synthetic growth methods for diamond were impactful innovations within the mid to latter-half of the 20th century, creating revolutionary materials development in several industries, such as the oil and gas, cutting and abrasive, end electronic industries, among others. Three of the primary sources for synthetic diamond that have been developed are high-pressure high-temperature (HPHT), detonation, and chemical vapor deposition (CVD). With respect to nanocrystalline diamond and fluorescent nanodiamond production, diamond of detonation or HPHT origin are the most important.
High Pressure High Temperature (HPHT) synthetic diamond is produced inside of high pressure (few Gigapascals) and high temperature (~3000 °C) cells which mimic the conditions of natural diamond growth (albeit much more controlled). In these cells, high purity graphite is transported through a molten metal catalyst (e.g. Nickel) to a diamond seed crystal where nucleation and subsequent growth of diamond will occur. Generally, particles on the order of several tens of microns to several hundreds of microns can be grown with the HPHT method. The biggest industrial use for HPHT diamond is in the preparation of highly wear resistant abrasive cutting or drilling components; however, HPHT diamonds are currently the primary source material for preparation of fluorescent diamond (for reasons which will be made clear in a moment).
Detonation Nanodiamond (DND) is formed in the extreme conditions that exist within the first few microseconds of an explosion. No, detonation diamond does not refer to diamond formed from detonating larger diamond particles to fragment them to smaller particles, instead, it refers to diamonds formed from the carbon contained explosives themselves. Explosives such as mixtures of TNT and RDX are detonated within a seal chamber, with cooling provided by gas, liquid, or even from ice in some cases (where the explosive charge is frozen within a block of ice before detonation). The high pressure and temperature experienced in the first few microseconds of the explosion caused the carbon contained in the explosives to convert to a liquid phase, and as the liquid cools, the carbon precipitates out as ~4-5 nm relatively spherical diamond particles. Many of the 4-5 nm “primary particles” often aggregate together, hence why detonation diamond is usually referred to as an “aggregate” size, unless the material has been sufficiently processed to isolate the 4-5 nm primary particles.
Chemical Vapor Deposition (CVD) Diamond is formed in special growth reactors at high temperature (but much lower pressure as compared to the HPHT or detonation techniques). The growth of diamond is initiated on a substrate containing seed diamond crystals using, typically, a mixture of methane (CH4) and hydrogen (H2) gas. CVD diamond and the methods of growing are extensions of growth methods used for many materials in the semiconductor industry; however, the reactors used for the growth of CVD diamond are distinctive from traditional CVD reactors (which will not be discussed here). CVD diamond allows for the most controlled growth of very atomically pure diamond, but it is not a scalable method of production for nanocrystalline particulate diamond. Nevertheless, CVD is an excellent means to produce thin film diamond for microelectronic applications, and it can be used to produce larger gem quality diamonds.
Type Classification of Diamonds
The most established scientific classification system of diamond relates to their either natural or synthetic origin, but to their nitrogen impurity content. Nitrogen is perhaps the most important impurity constituent in diamond because of the dramatic effects it can have on the optical appearance and fluorescence properties. It should be noted that the term ‘impurity’ in this sense means ‘not carbon’ and does not implicitly mean the presence of the nitrogen is an unwanted contaminant (in some cases, this may be true, but in other cases, it may not be). The two major type classifications are:
- Type I: Diamond which contains Nitrogen as its primary impurity constituent.
- Type II: Diamond which contains almost no measurable nitrogen impurities.
Within the Type I classification:
- Type Ia: Diamond containing approximately 0.1 at.% (~1000 ppm) of substitutional nitrogen impurity content. Almost all natural diamonds are of Type Ia.
- Type Ib: Diamond containing approximately 0.01 at.% (~100 ppm) of substitutional nitrogen impurity content. Most HPHT diamonds are of Type Ib.
Similarly, the Type II classification contains two sub classifications (Type IIa and Type IIb); however, they are not currently utilized in the production of fluorescent nanodiamonds. Most CVD synthetic diamond is of Type II, and contains less than 0.005 at.% (<50 ppm) of nitrogen impurities. The presence of boron is used to determine the distinction between Type IIa and Type IIb, with Type IIa being the most atomically pure diamond.
